Can deep ultraviolet decompose formaldehyde?
There are two kinetic processes in the decomposition of formaldehyde by UV: one is that the photolysis is interrupted by UV directly; the other is that the UV reacts with water in the air to produce OH radical to decompose formaldehyde. (mercury lamp tube is OK). Deep ultraviolet can directly decompose formaldehyde and other organic pollutants by breaking the chemical bond of formaldehyde.
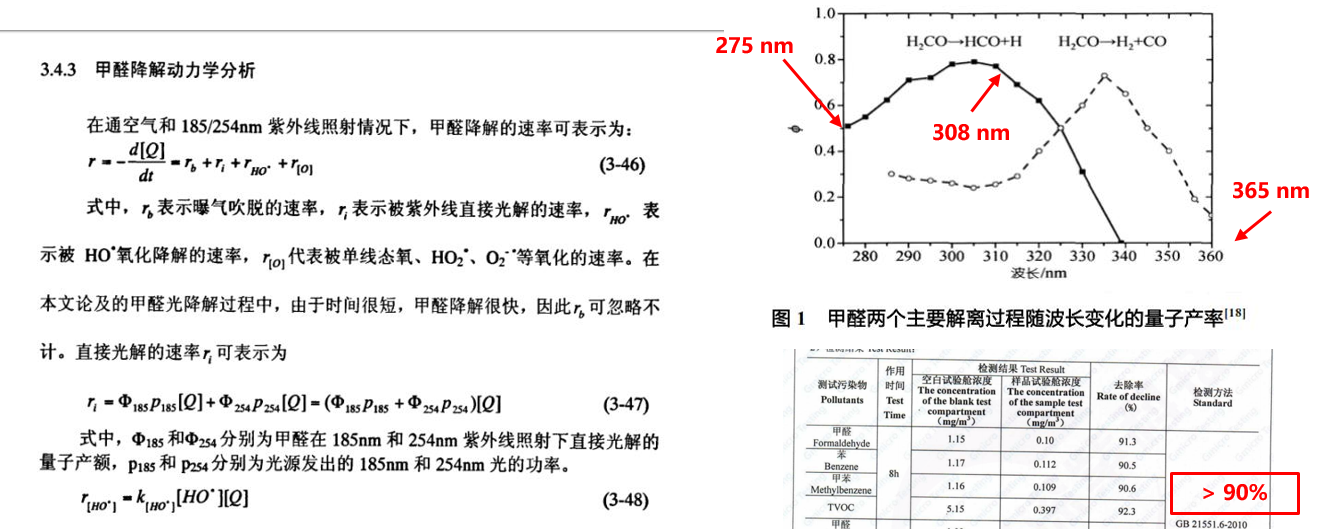
Can deep ultraviolet ray decompose formaldehyde and other organic pollutants? In fact, many people think that it should not work, but in fact it can. The bond energy of O-O bond is 5.1ev corresponding to 243nm, but the necessary C-H bond energy in organic compounds is 4.31ev, corresponding to 283nm, C-O bond energy is 3.39ev, corresponding to 366nm. If the photon energy of ultraviolet is higher than the bond energy, it can be interrupted, just as it destroys DNA molecular chain. Therefore, UV can decompose formaldehyde.
However, we should also note that the decomposition process of formaldehyde is very complicated, and its two decomposition processes are directly related to the wavelength. The quantum yield changes of formaldehyde decomposition at different wavelengths. Although we don't know what two peaks are at 305 nm and 340 nm, there is no doubt that UV can decompose formaldehyde by itself. Compared with toluene and TVOC, UV can decompose.
